Today in Chemistry, we learn about covalent bonding.
Unlike in ionic bonding, the atoms cannot transfer electrons to one another so
they have to share electrons. An example is Cl2 or
Cl-Cl.

Sometimes there can be a double or
triple bonding. An example is N2. Instead of having one line between the two
nitrogen. Instead there is 3 lines because it is triple bonding.
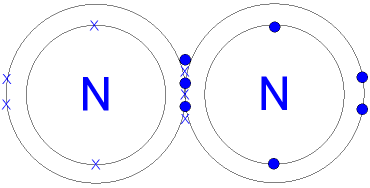
Compounds can contain more than one
covalent bond. An example of this is methane or CH4 which needs 4
hydrogen. It will be written as 4 lines surrounding C with H in it.

No comments:
Post a Comment